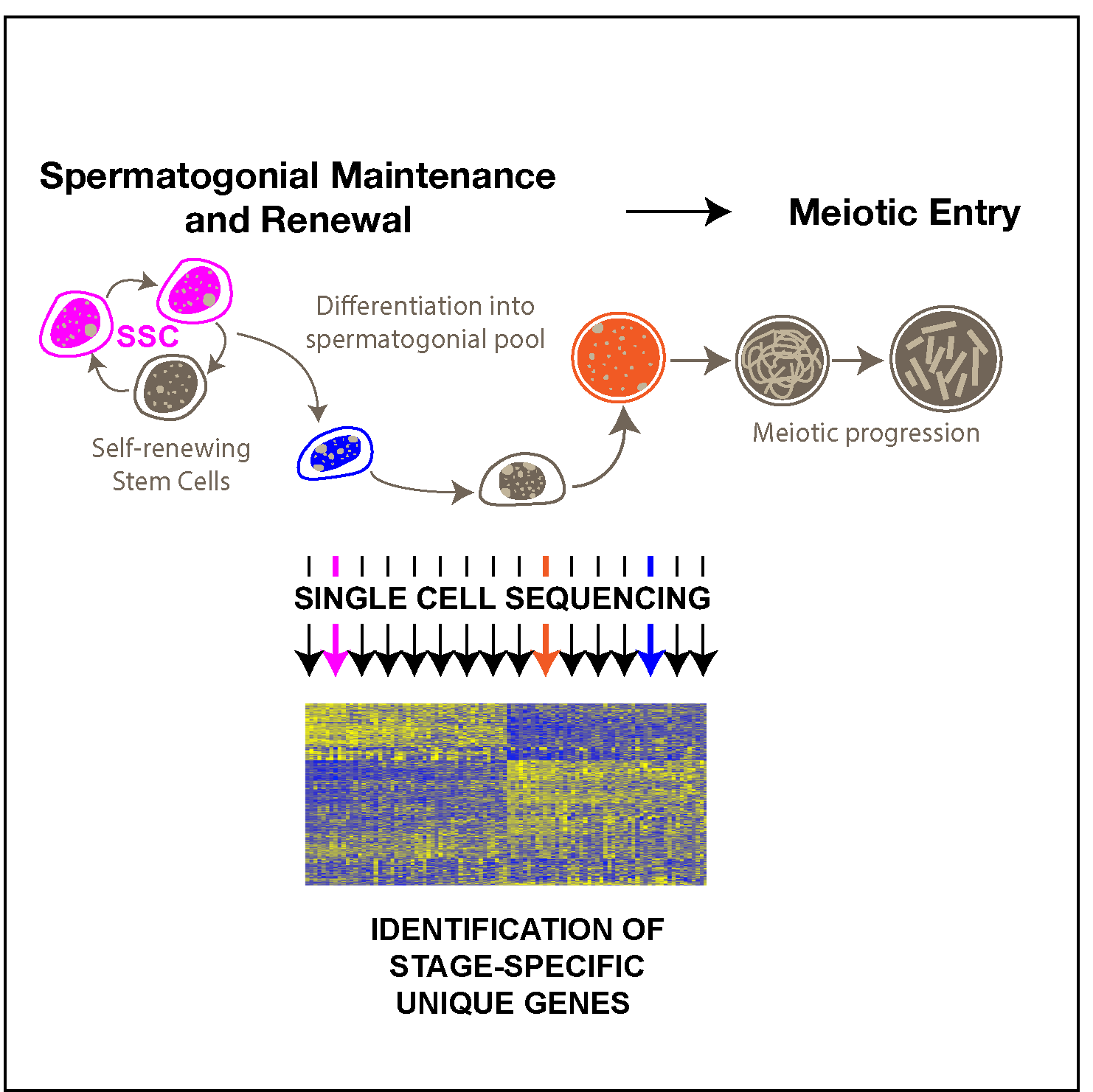
RESEARCHING THE MYSTERIES OF THE MAMMALIAN GERM CELL

Studies in the Cohen lab span the entirety of spermatogenesis, asking a simple question: how are the unique events and transitions of spermatogenesis and oogenesis regulated at the genetic level to ensure the production of healthy viable gametes. We are particularly interested in the events of meiosis since it is during meiotic prophase I that numerous errors can arise that result in the production of gametes that are either unable to give rise to a healthy embry or that result in premature miscarriage or birth defects in humans. Indeed, some 40-60% of all human conceptuses are thought to have the wrong number of chromosomes due to errors in meiosis. Thus, while the lab studies virtually all aspects of spermatogenesis and oogenesis (see projects outlined below), we are particularly intrigued by meiosis.
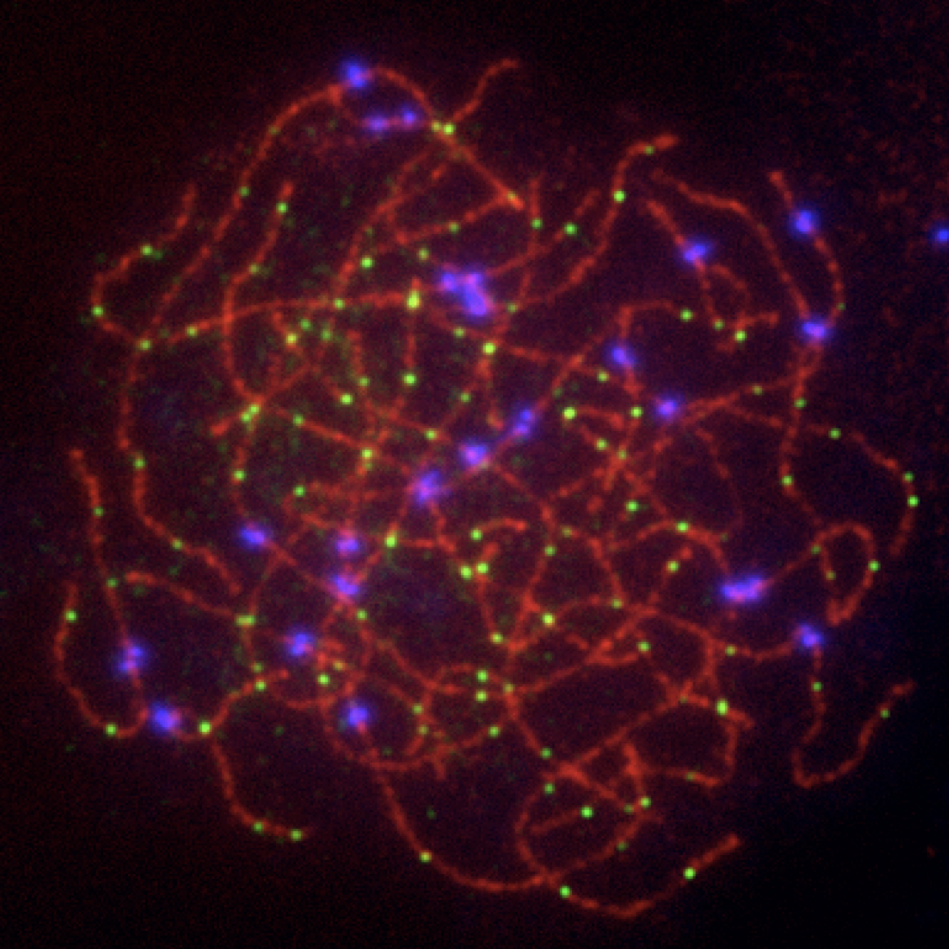
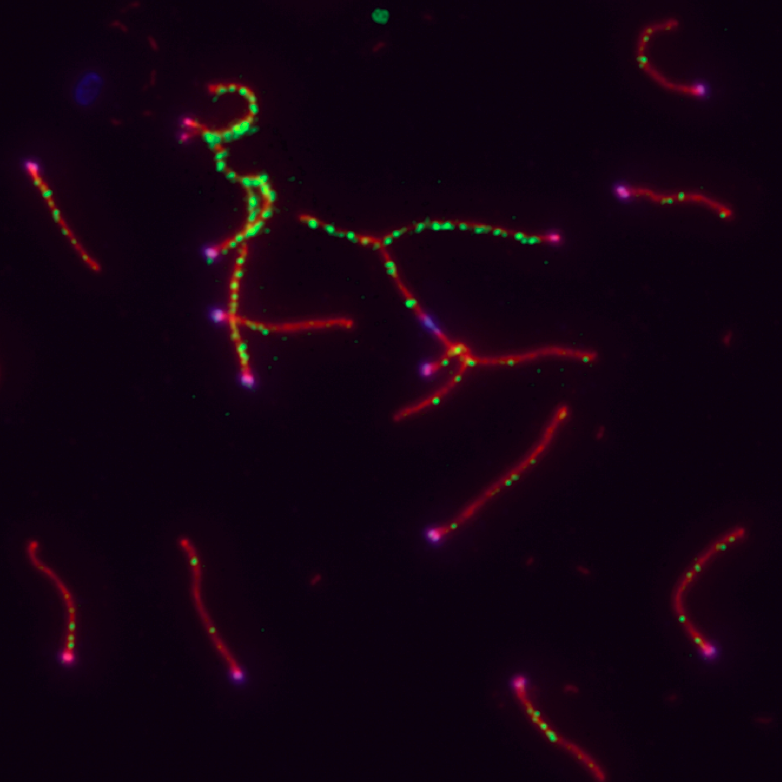
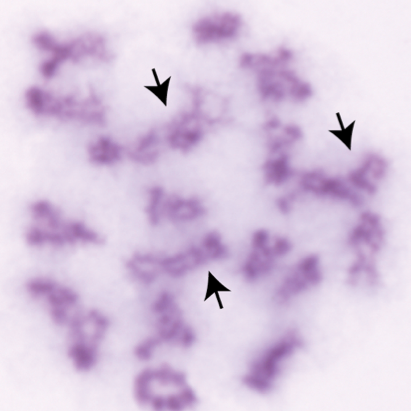
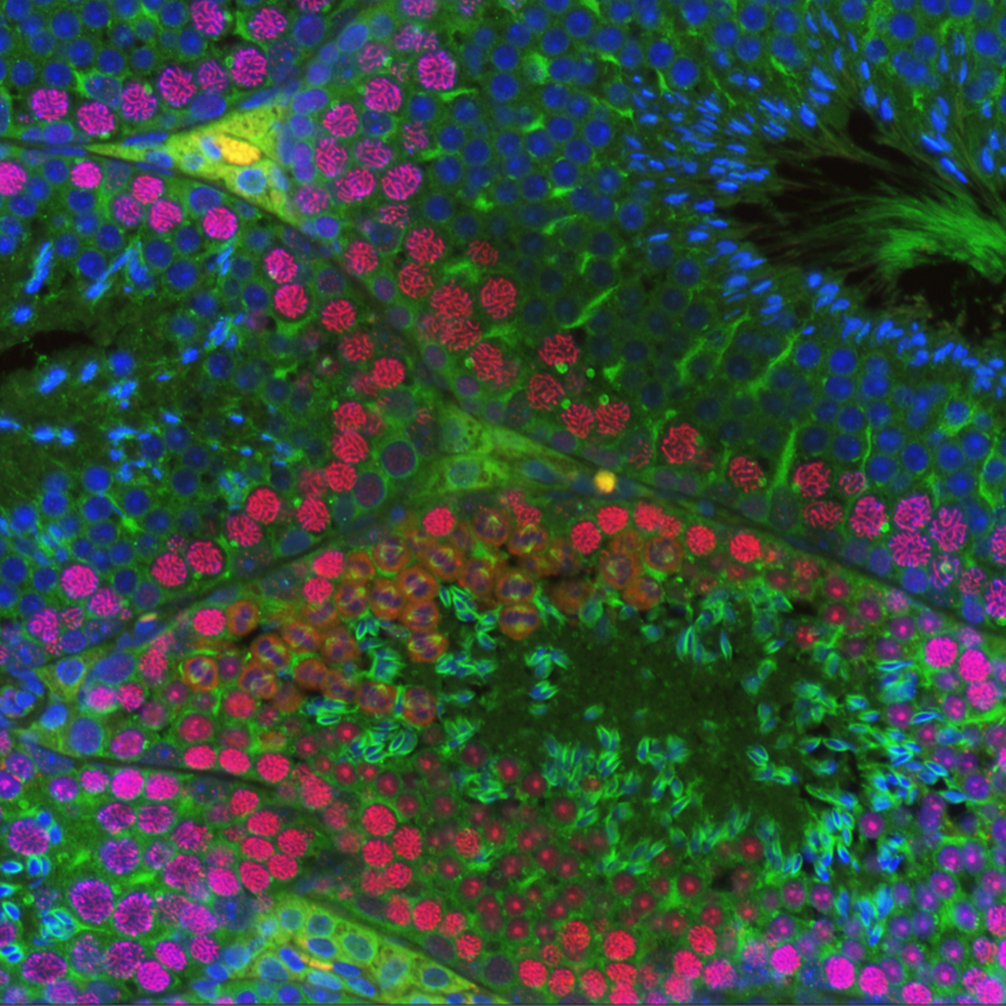
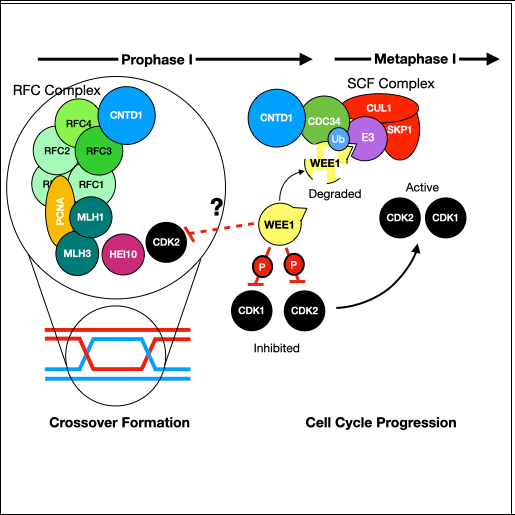
In early meiotic prophase, SPO11 creates hundreds of DNA double-strand breaks (DSBs) throughout the genome. Spermatocytes and oocytes repair these DSBs via homologous recombination, producing either crossovers (sites of physical exchange of DNA between parental chromosome pairs) or noncrossovers. Crossovers serve two essential purposes: 1) Shuffling alleles into new combinations, facilitating genetic diversity in a population; and 2) Physically tethering homologous chromosomes together, ensuring their proper segregation into separate daughter cells. It is critical that at least one crossover forms between each chromosome pair as a safeguard against abnormal numbers of chromosomes (aneuploidy) in the resulting gametes – and ultimately offspring. The frequency, timing, and placement of crossovers are highly regulated and are sex- and species-specific. For instance, human and mouse males have significantly lower crossover numbers than females, and males preferentially form distally-placed crossovers. Although DSB number is sexually dimorphic in humans, the frequency is similar between male and female mice. In both species and sexes, only a small proportion of DSBs are resolved as crossovers (~10% in mouse; 28 - 37% in humans). Of the DSBs that form crossovers, a large majority (90-95%) are resolved via the class I crossover pathway, which utilizes MutS-gamma (MSH4/MSH5) and MutL-gamma (MLH1/MLH3). A second, minor class II crossover accounts for the remaining 5-10% of crossovers. In this latter pathway, the structure-specific nuclease MUS81 works in concert with BLM helicase to resolve complex multichromatid join molecule intermediates.
Meiosis
Since 2000, the Cohen Lab has studied meiotic recombination and the events of prophase I in mammals, and has been funded by NIH throughout this time. Our studies focus on the class I and class II crossover pathways, and their regulatory control/interactions.
DNA Repair Pathways
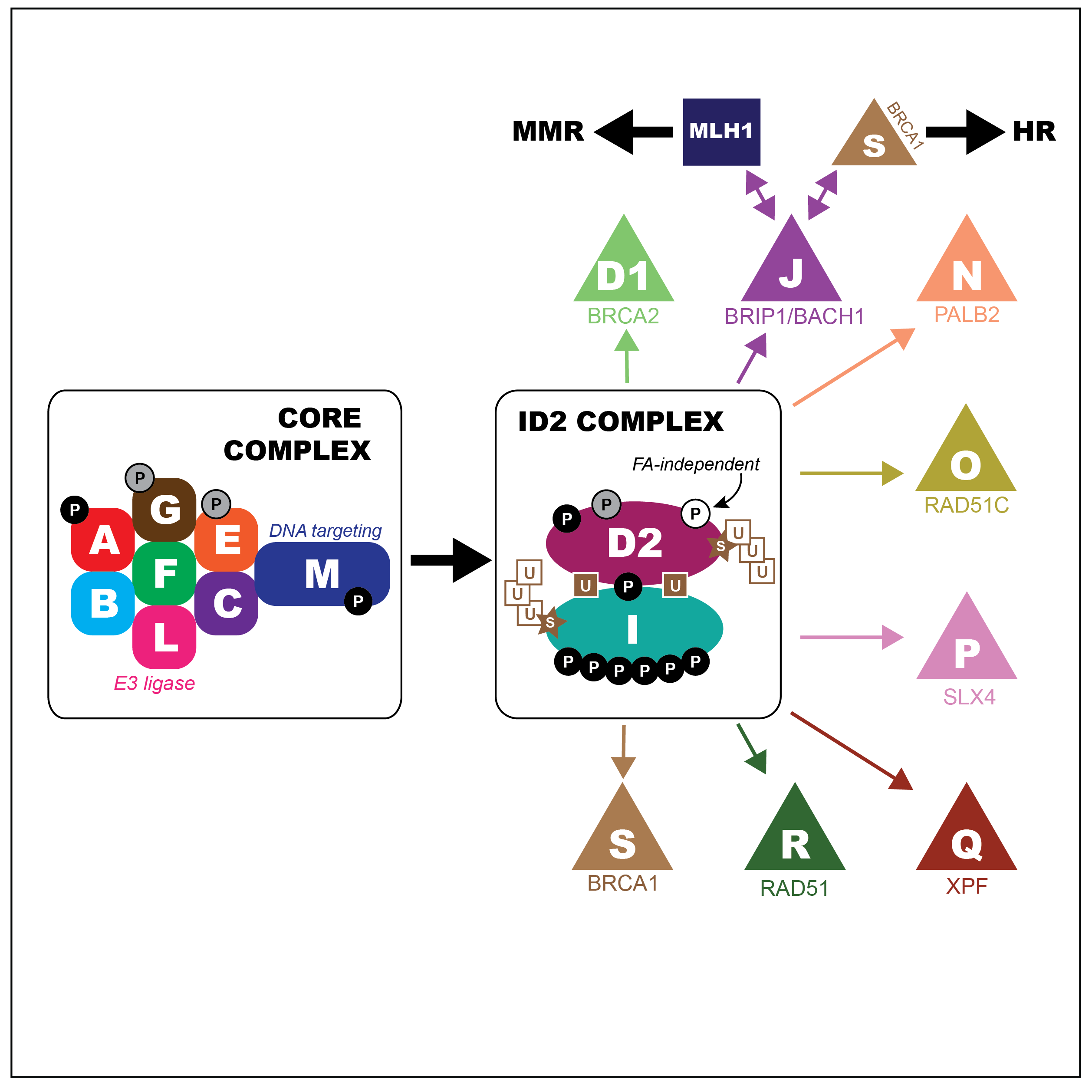
Our studies focus on the DNA mismatch repair pathway, the MUS81-EME1 structure specific nuclease, and the Fanconi Anemia pathway. We study these pathways in the context of meiotic recombination as well as in the context of genome stability in the mammalian germ line.
Gene Regulation
We seek to understand the complex gene regulatory environment that exists throughout spermatogeneis, from transcriptional regulation, to the role of non-coding RNAs in post-transcriptional regulation, and to the post-translational regulation of protein function.
Contraception
Studies in the lab are focused on identifying intervention points through spermatogenesis that could be targets of contraceptive drug targeting. Our goal is to provide cheap, safe, and reliable contraceptive options for men. Funded by the Bill and Melinda Gates Foundation.