
Mouse CNTD1 exists as a smaller size protein that lacks the capability of binding to CDKs in vivo
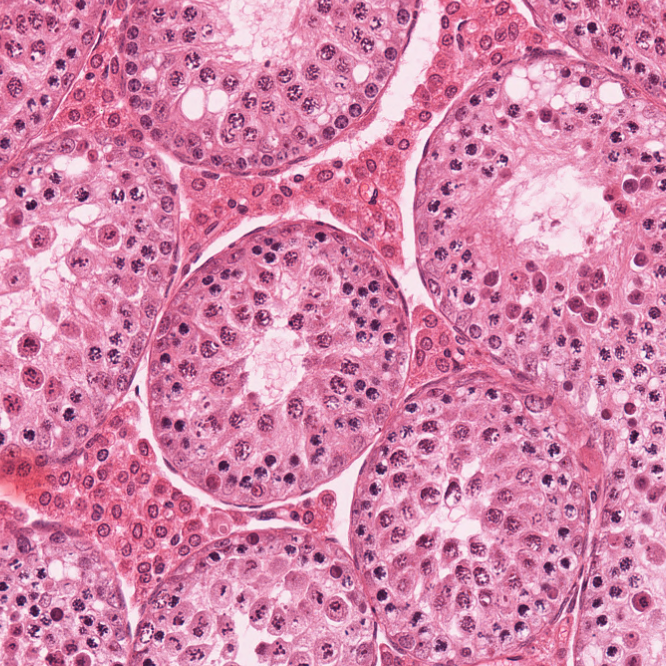
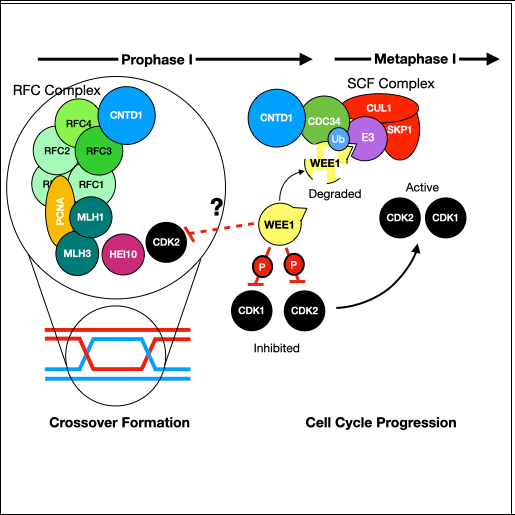
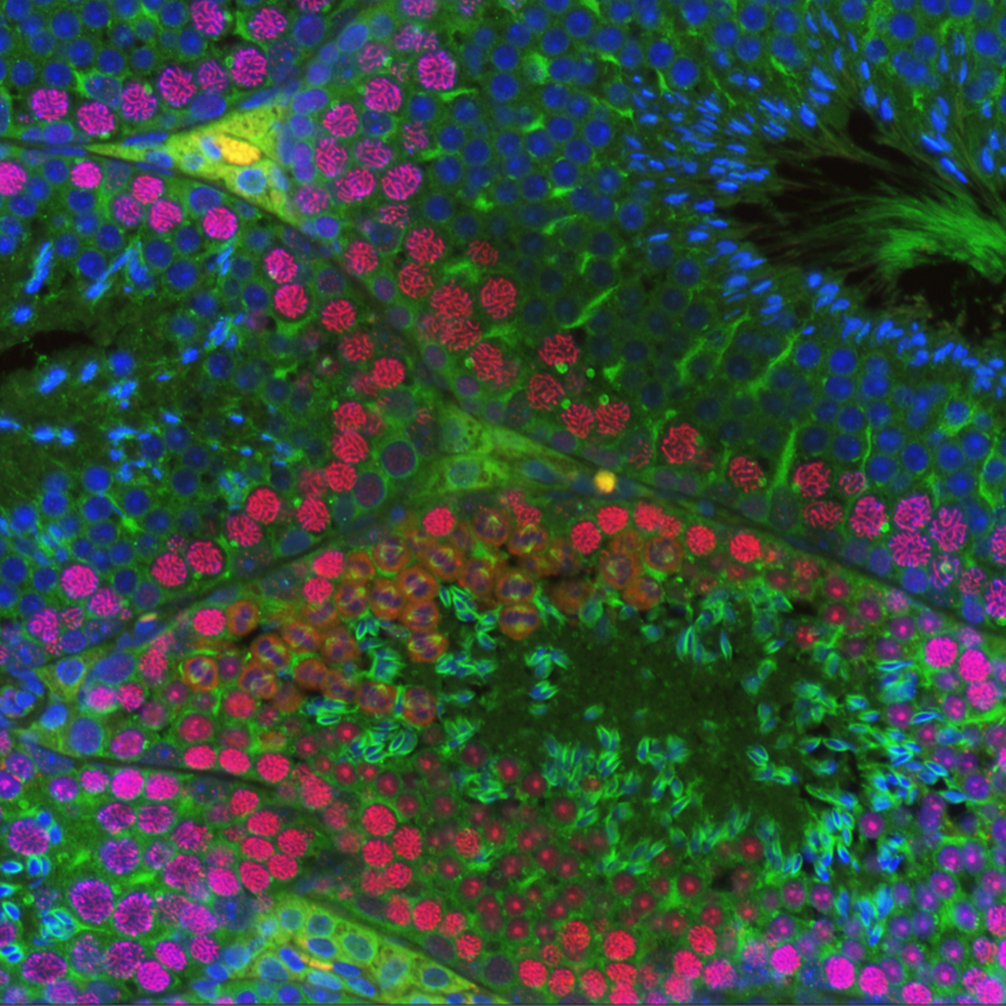
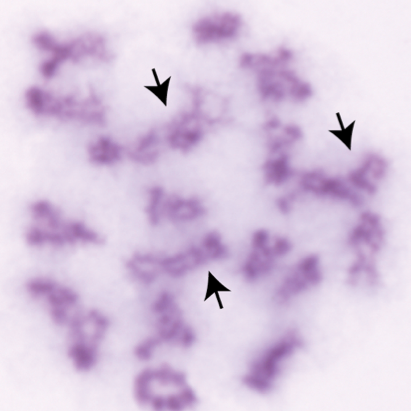
The lab's most recent work on CNTD1 was published in Cell Reports on July 7th, 2020. The work was carried out by Dr. Steve Gray, a former postdoc. Steve made a dual tagged allele of Cntd1 by CRISPR/Cas9 and was able to demonstrate localization of CNTD1 to nascent crossover sites during pachynema of prophase I. Interestingly, Western blot analysis of the tagged protein revealed a smaller than expected protein for endogenous CNTD1, while yeast two-hybrid, immunoprecipitation-western blotting, and mass spectrometry all indicated that this short form CNTD1 does not interact with any known cyclin-dependent kinases. Instead, endogenous mouse CNTD1 appears to interact with two functionally relevant protein complexes during prophase I: the Replication factor C (RFC) complex and the SKP1-Cullin-Fbox (SCF) complex. Through these interactions, we propose that CNTD1 controls both crossing over (by regulating MLH1-MLH3 function through RFC-PCNA interactions) and cell cycle control (through ubiquitylation by SCF of the key cell cycle regulator, WEE1).