Conserved and overlapping functions of the Argonaute proteins in spermatogenesis
Non-coding RNAs (ncRNAs) play crucial roles in post-transcriptional regulation of gene silencing/expression in spermatogenesis [1]. MicroRNAs (miRNAs) and short interfering RNAs (siRNAs) are two major classes of small ncRNAs, ranging in size from 20 to 24 nucleotides. Both exert their regulatory function by associating with members of the Argonaute protein family and coordinate downstream post-transcriptional gene-silencing events. Proteins from the subclass AGO represent a highly conserved gene family that is found in bacteria, archaea and eukaryotes [2], yet we have little information regarding how they regulate gene expression at the transcriptional and post-transcriptional levels in the mammal germline. Canonically, these proteins are found in the cytoplasm where they associate with small ncRNAs to form the RISC complex (RNA-Induced Silencing Complex) and silence target mRNAs. However, evidence suggests that AGO–RNA complexes can also regulate nuclear events such as transcription, genome maintenance and splicing [3]. Although the four AGO proteins found in mammals (AGO1-4) are structurally very similar, only AGO2 has retained the ability to slice, or catalytically disable target mRNAs, even though all four AGOs retain the PIWI domain, essential for the target cleavage. Notwithstanding, regulation of expression of target mRNAs by AGO–RNA is primarily accomplished without cleavage, raising the question about silencing mechanisms of the AGOs 3,1 and 4 in mammals.
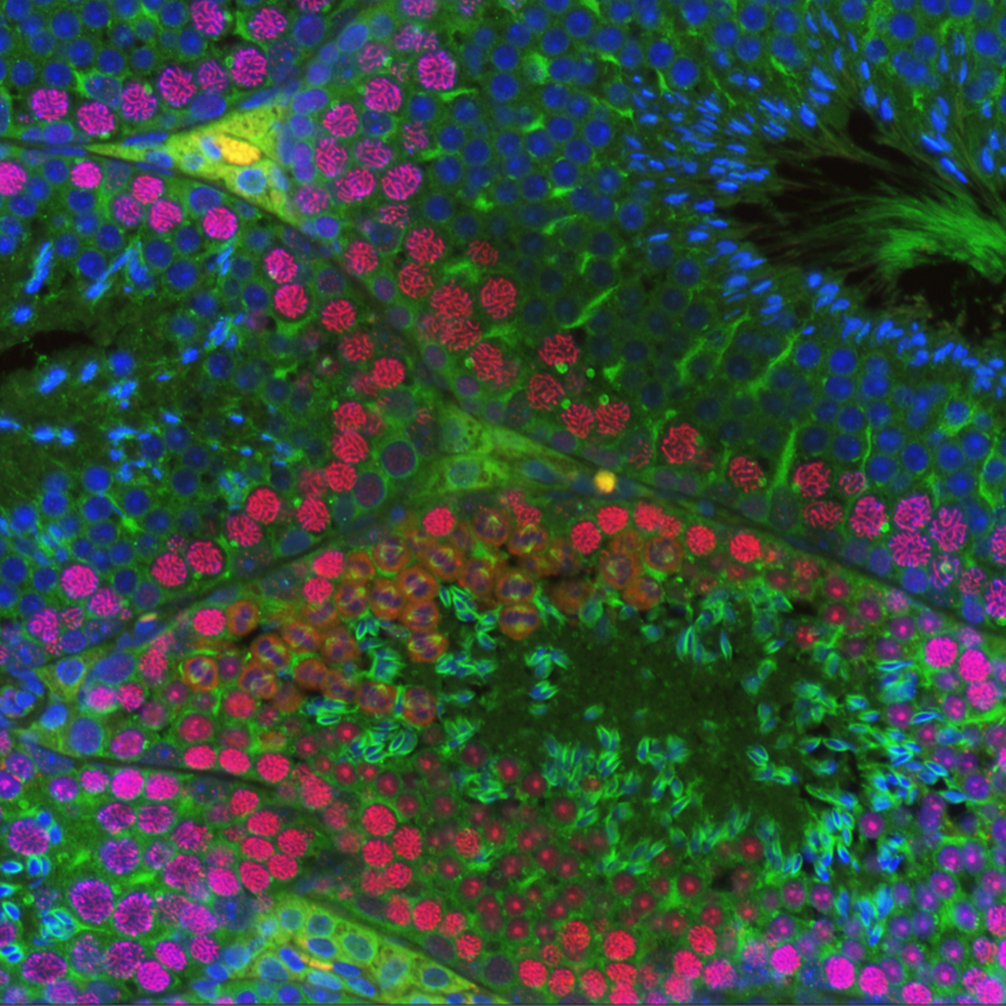
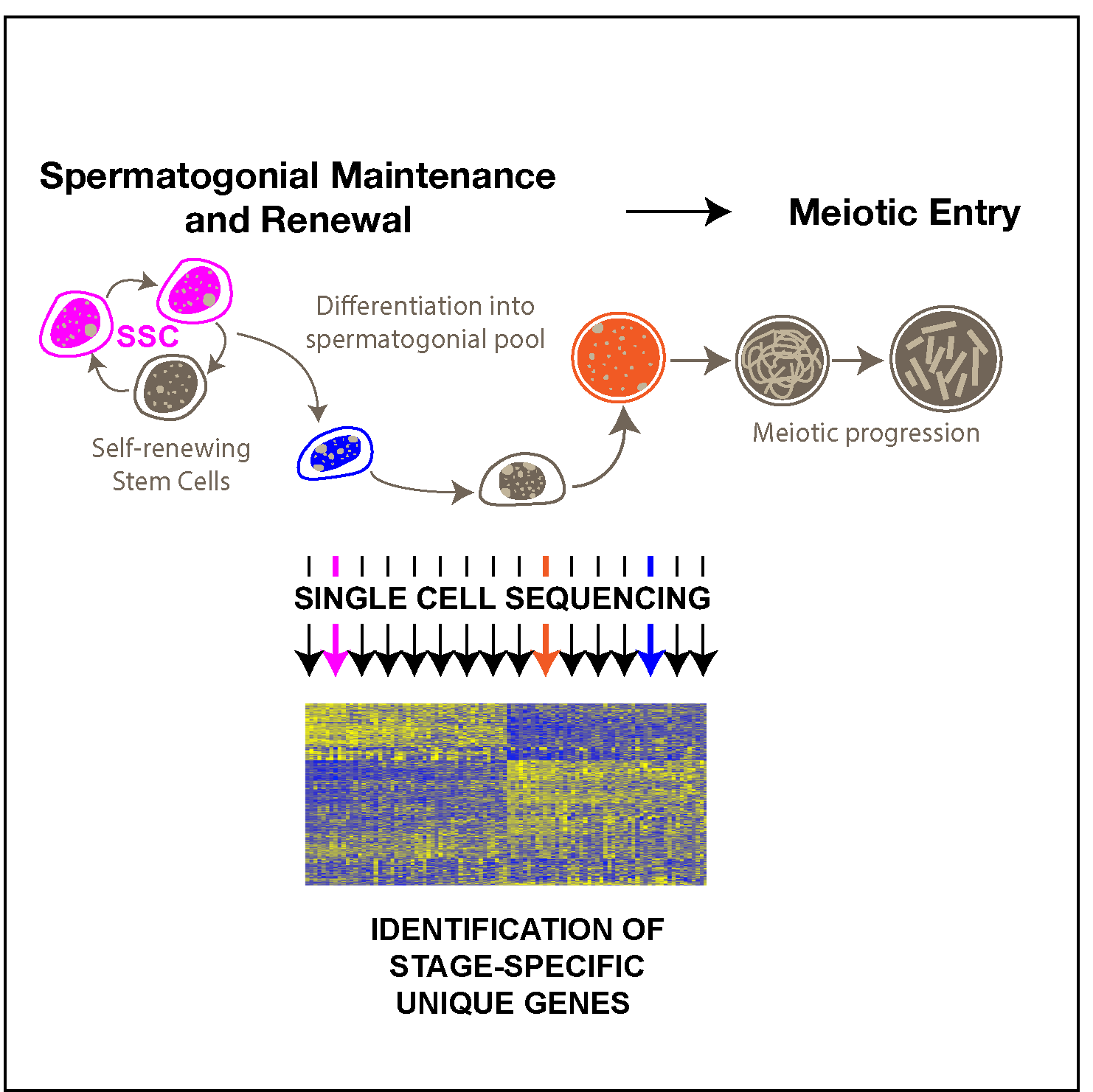
Our research on AGO proteins began with the observation that AGO3 and AGO4 are highly expressed in mouse spermatocyte. We have demonstrated that these proteins reside in the nucleus of the male germ cell, with somewhat overlapping, but also distinct patterns of localization [4]. Both, AGO3 and AGO4 localize in the nuclei of pachytene spermatocytes, at sites of asynapsis and in the transcriptionally silenced XY subdomain named the sex body (SB). Using an Ago4 deficient mutant mouse line, we showed that AGO4 plays a role in transcriptional silencing during meiotic prophase I and that loss of Ago4 results in a compensatory increase in Ago3 expression. However, lack of AGO4 results in dramatic loss of small ncRNAs, more than 20% of which arise from the X chromosome. Thus, these studies indicate that AGO3 and AGO4 may play critical roles in the regulation of gene silencing in meiosis, but that this function resides at the level of the nucleus, and thus may involve transcriptional control rather than the more traditional role of AGOs in post-transcriptional mechanisms. More recent preliminary data has shown that when Ago1, 3 and 4 are lost, mice exhibit a subtle fertility phenotype characterized by decreased sperm count, testis size, and most curiously, a distortion in the expected 1:1 sex ratio of offspring from these mice. These observations raised our interest in the role of non-slicing Argonaute proteins and their small ncRNA cargoes as key regulators of gene expression that drive the production of healthy sperm.
REFERENCES
[1] Chu and Shakes. Adv Exp Med Biol. 2013;757:171-203. [2] Höck and Meister. Genome Biol. 2008;9(2):210. [3] Gagnon and Corey. Nucleic Acid Ther. 2012;22(1):3-16. [4] Modzelewski et al. Dev Cell. 2012;23(2):251-64.
Summary prepared by Dr. Maria de Las Mercedes Carro