Paula has been honored with the prestigious SSR Trainee Mentoring Award from the Society for the Study of Reproduction (SSR)...
-
The Cohen Lab
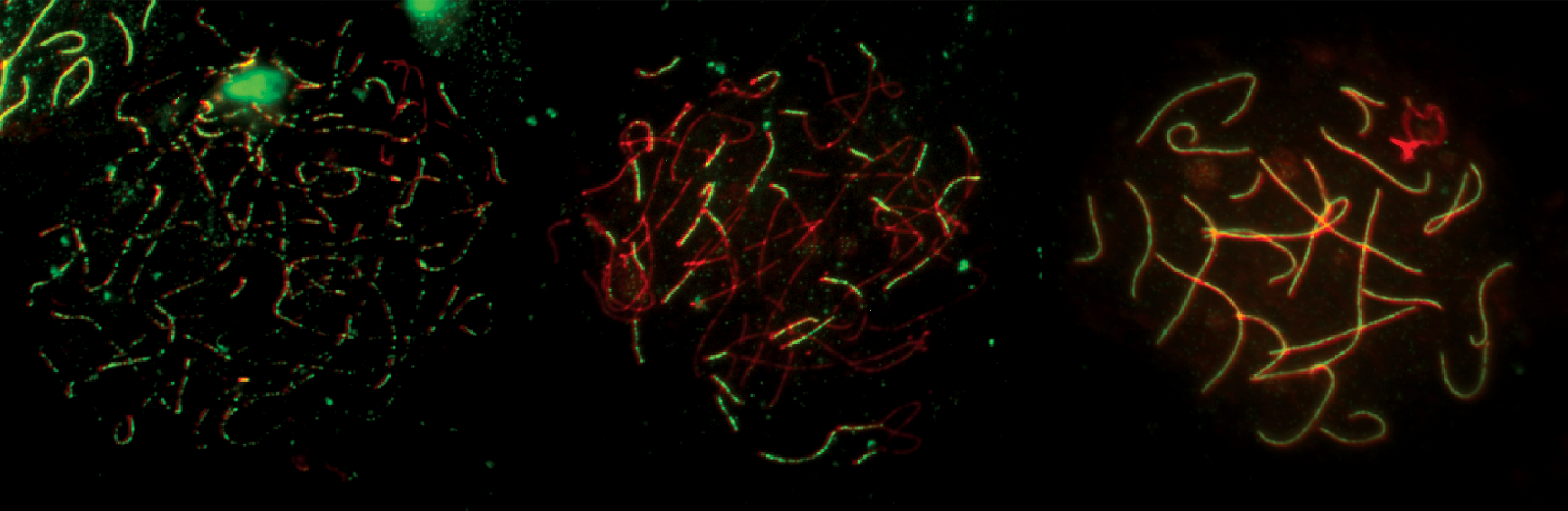
Investigating the various pathways that lead to crossing over during mammalian meiosis,
Learn more
and exploring the interactions between these pathways.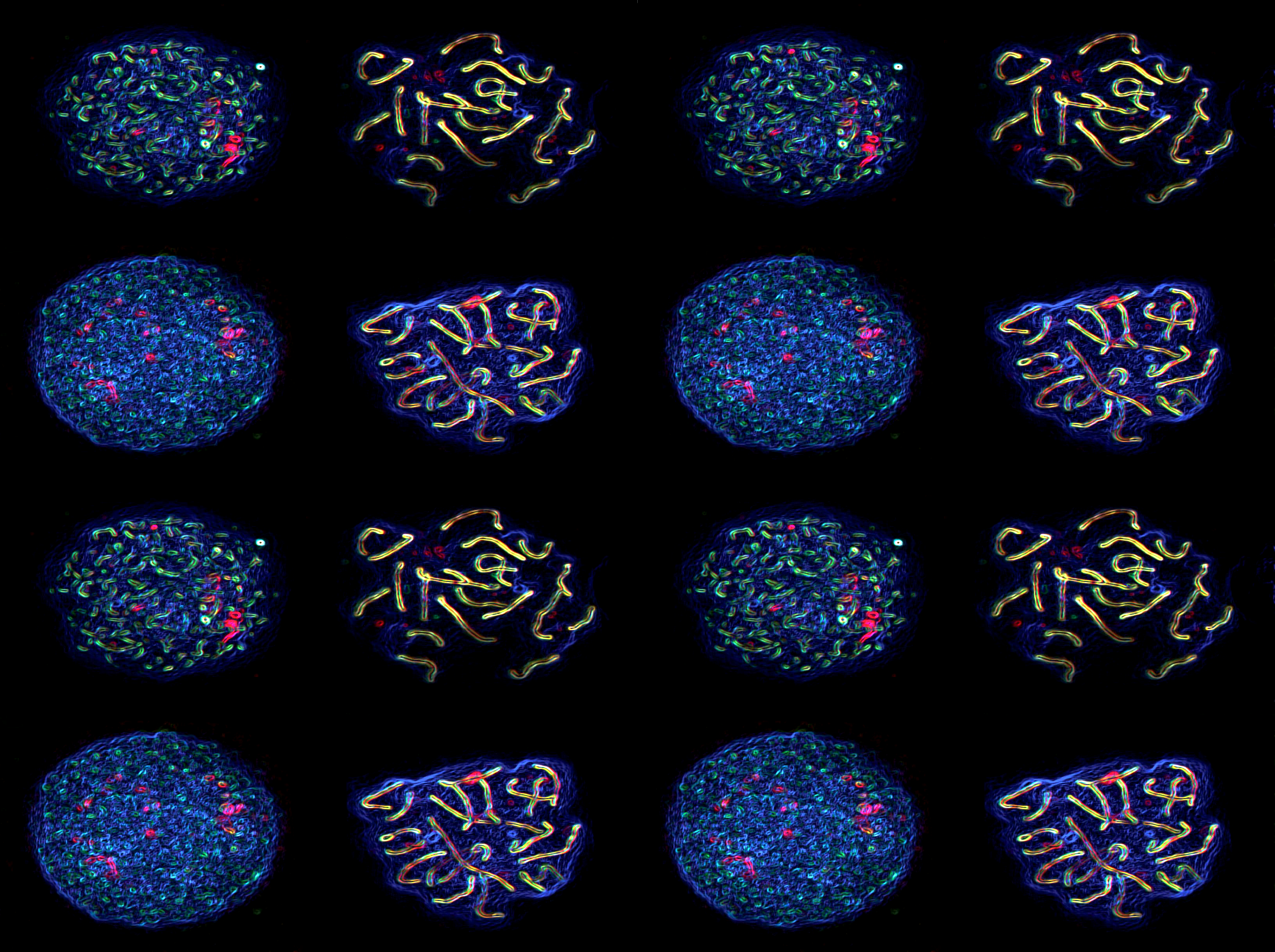
-
The Cohen Lab
Establishing the molecular signals that enable spermatogonia to
Learn more
become competent to enter meiosis.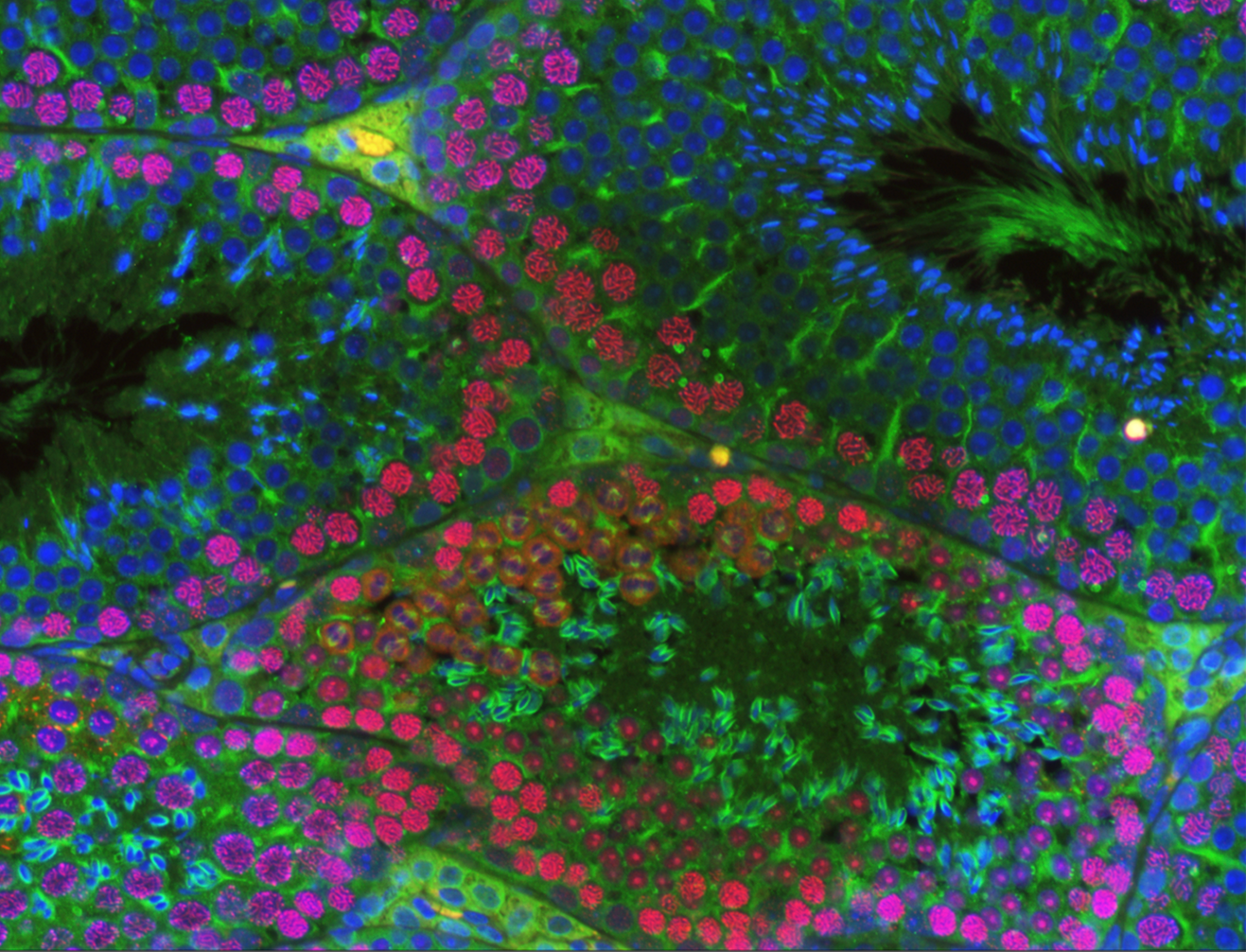
-
The Cohen Lab
Elucidating the role of the Argonaute proteins in meiotic prophase I and beyond,
Learn More
and identifying their critical RNA cargoes during spermatogenesis.